I think people in the future could use this information for housing or maybe the city council could use this information by measuring the pH of rain water and seeing how it could rust houses nail's or maybe the need to design something requiring lots of iron and so to see how long it could last for.
Conclusion
For my conclusion I had finally got my answer. My hypothesis was correct since the nail in the vinegar hadn't even rusted at all, the nail in the sprite had completely rusted all over and finally the nail in the soda water had white patches which I found out is soda powder and had the bit of rust on at the bottom which seemed to be different to the rust on the nail in sprite, it looked like it had rusted for years since it was very red. but the other parts remained clean as if they were new. I then also discovered why the sprite had rusted faster then the4 soda water, it was because of the air bubbles that had oxegen inside them. What actually made nails rust actually the electrons inside the liquids which can be enhanced when it comes in contact with oxegen. I could have improved the experiment by adding a few more alternatives and testing them. So overall it left with soda water having the pH of 8 having the 2nd most rust, but greater intensity of rust. The nail with the most rust was the nail in sprite with the pH of 5 and the nail with no rust at all was the one in vinegar with the pH of 2.
Posted by Hongy Boi 0 comments
Day 10 results

For todays final results, it ended up like:
-The nail in the sprite had completely rusted (5 pH)
-The nail in the soda water (8 pH)
-The nail in the vinegar hadn't rusted at all (2 pH)
Posted by Hongy Boi 0 comments
Day 9 results

Today's results were the same now, the nail in the sprite had rusted and the nail in the soda water had completely been covered in white patches, finally the nail in the vinegar.
Posted by Hongy Boi 0 comments
Day 8 results

For todays results there wasn't much change at all except for the soda water nail. There was a red colour on the bottom, which was probably rust, but the nail else where remained clean.
Posted by Hongy Boi 0 comments
Day 7 results

The rust has completely covered the nail in the sprite and its bubbles had gone as well. The soda water nail seemed to have completely covered it in patches as well. The nail in the vinegar hadn't changed at all.
Posted by Hongy Boi 0 comments
Day 6 results

The nail in the sprite had completely rusted and was black all over. The rust had covered a layer of the nail and the for the nail in soda water, you couldn't see much rust since it was covered in white patches.
Posted by Hongy Boi 0 comments
Day 5 results

Todays results weren't much different to the ones yesterday, the nails hadn't rusted much at all. The white patches on the soda powder nail did begin to rust a bit though.
Posted by Hongy Boi 0 comments
Day 4 results

Today results was a major difference, the nail with the highest pH ( sprite) had at least half of the outside rusted. The soda water had made big chunks cover all of the top 1/4 of the nail. The nail in the vinegar remained the same though.
Posted by Hongy Boi 0 comments
Day 3 results

This day I found a white starchy looking substance covering a part of the nail in the liquid with the pH of 5 (soda water). Then I found out it was actually the soda powder which was covering the nail. The rust on the nail in the sprite was growing. Yhere will still no signs of rust though on the nail which is in vinegar.
Posted by Hongy Boi 0 comments
Day 2 results

Day 2 results, I didn't much signs of rust on any of the nails, but the 8 pH liquid which is the sprite did have traces of rust on the bottom
Posted by Hongy Boi 0 comments
Day 1 results

Day 1 results:
No signs of any rusting in any of the containers. Tipped some liquids out since corroding needs to have oxygen for the electrons to travel and corrode the substaance faster.
Posted by Hongy Boi 0 comments
Method

Firstly set your containers on the same surface, room/ temperature. Prepare the liquids onto a table in measuring cups. Then take out your pH scale and find out the acid levels. Next place the iron nails into the containers and measure the liquids to make sure they're the same amount. Make sure you know which liquid is which and pour them into the separate containers, then label the containers since its hard to tell which is which. Do not touch or turn the containers since it may mantle with the corroding processes. Look at the time to see when you poured in the liquids (vinegar, soda water, sprite/ fizzy) and so you know when to look back at the nails. After 1 day record what has happened to the nails and take a photo or explain it thoroughly. Keep this routine up for about 15-20 days and see what has happened.
Posted by Hongy Boi 0 comments
WAIT!

Sorry! I accidentally added 'Lemon'as part of my equipment. I had just tested and found out that vinegar has the same pH level as lemon juice. So I've decided to change it to sea water so it doesn't have the same pH level as the others.
Posted by Hongy Boi 0 comments
Equipment/ Apparatus
You will need like I said:
Vinegar
Soda Water
Fizzy Water ( sprite)
3x Containers with the same capacity

1x pH scale

3x Nails
1x Log Book to record the results
Posted by Hongy Boi 0 comments
Variables to be testest

I will change one container to vinegar, one to tap water and one with lemon juice. I will then measure the pH levels of the liquids and see how it will affect the corrosion process. I will then research more on about it and how it does affect it.
Posted by Hongy Boi 0 comments
My Hypothesis

I think that the the nail that is being soaked in the lowest pH level liquid will make the nail rust faster and will completely cover the nail in rust. I think this because lemons have a strong taste and will make the and has been proven that a lemon can preserve decay on a apple. So I think the liquid
Posted by Hongy Boi 0 comments
This is how I will make sure I have developed a fair test

I will make sure that the containers have the same capacity as the rest and are the same shape and size. I will have the same amount of millilitres of liquid and the nails will be the same brand, length and type (iron). I will drop the nails into the containers at the same time and will keep the containers in the same room so that temperature doesn't affect the containers. I will also make sure that I will record the nails corrosion.
Posted by Hongy Boi 0 comments
This is how I will test my question

I will see what kind of acid has the most affective effect on a nail. I will do this by getting 3 containers the same compacity and add the same amount of millilitres of a acidic substance into the containers. After I have added the liquids, I will measure the pH levels of the acid, then put in an iron nail into the containers. I will then record the amount of corrosion corrosion the acid had caused. I will record it for 20 days.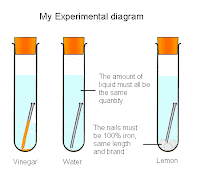
Posted by Hongy Boi 0 comments
My Investigative Question
My science question is:
How does hydrochloric acid affect the way a nail rusts? Why does it happen?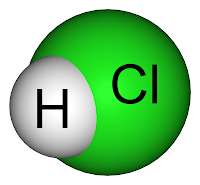
If you don't know what hydrochloric acid is, it's a solution of hydrogen chloride (HCl) in water. It is a highly corrosive, strong mineral acid and has major industrial uses. It is found naturally in gastric acid. During the Middle Ages, it was used by alchemists in the quest for the philosopher's stone.
Posted by Hongy Boi 0 comments
Ad
Hey, if you want to know about the latest naruto then go to my second
blog because this is my science fair homework blog and I don't want it to
affect my marks.The address is:
narutoninjastorm.blogspot.com
Posted by Hongy Boi 0 comments
I've pinned it down!
I've already decided which science experiment i'm going to do!
I'm going to do the old acid and rusting topic! I'll go through the processes
that you need to go through later, but first i'll tell you a hint.
It's got something to do with nails/iron.....
I'll tell you what you need first:
3 iron stuff/nails
3 jars
1 lemon
1 ph scale
vinegar
tap water
Thats all I'll tell you for now............ and i'll tell you more later.
Posted by Hongy Boi 0 comments
My Science concept model

I'm doing my concept model on the the leaning tower of pisa because it demonstrates the concept of center of gravity and stability of body
Posted by Hongy Boi 0 comments
2nd day
yo, This is the second day, not only will I do my experiment blogging here, but also I'll keep you up to date with the new naruto.
Posted by Hongy Boi 0 comments
Welcome

Hi guys.....
nuthin much 2 say reely so a quick tntro wil do.
Science exp. Doin mine on ph acids and rust.
Hope u enjoy!
Posted by Hongy Boi 2 comments






